Professor
jherndon@nmsu.edu | (575)-646-2487

- B.S. Chemistry, University of North Carolina-Greensboro, 1979
- M.S. Chemistry, Princeton University, Princeton, NJ, 1980
- Ph.D. Chemistry, Princeton University, Princeton, NJ, 1983
Research
Research in the Herndon group focuses on the discovery of new organotransition metal-based processes for use in organic synthesis. Of specific interest are reactions that transform simple starting materials into complex products in a general, predictable, efficient, and stereoselective manner. Most of our present activity involves exploration of the coupling reaction between Fischer carbene complexes and highly conjugated acetylene derivatives. Several useful processes have been identified from these studies, and two of the systems under study are detailed below.
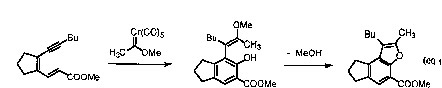
The coupling of Fischer carbene complexes with enyne-aldehydes results in furan derivatives (eq 1). The unstable isobenzofuran ring system can be generated using benzo analogs (eq 2). Although unstable, this ring system very predictably undergoes Diels-Alder reactions. This reaction sequence serves as the cornerstone for preparation of pharmaceutically important structures (e.g. the estrogen-like structure depicted in eq 3) from two relatively simple components.
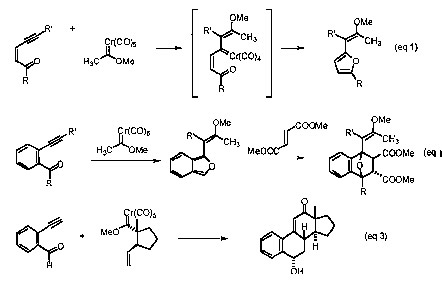
The coupling of Fischer carbene complexes with dienynes results in the synthesis of the benzofuran ring system (eq 4). This process has been found to be very and general and tolerant of a variety of substitution patterns. Numerous medicinally important compounds contain the benzofuran ring system and current research activity involves the synthesis of biologically active compounds for medicinal evaluation.